The self-repair mechanism of stainless steel
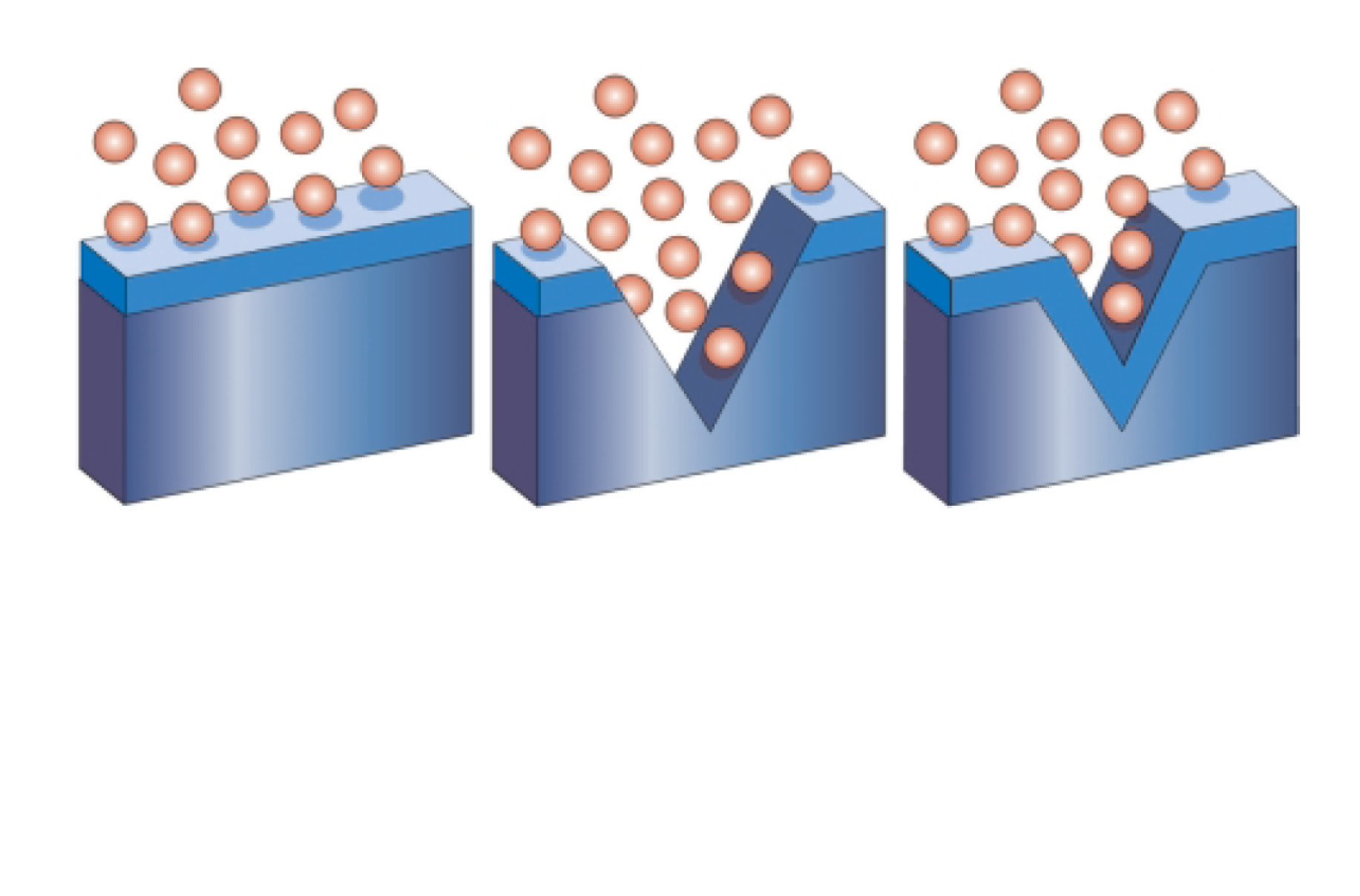
Stainless steel is self-healing. This is why it does not require any coating or other corrosion protection.
Download the information hereStainless steel is well-known for its corrosion resistance. More information on the corrosion properties of stainless steels can be found here.

Stainless steel is self-healing. This is why it does not require any coating or other corrosion protection.
Download the information here
Material decay and corrosion are natural processes. Almost all materials will in one way or another decay over time. The effects of corrosion in our daily lives can be seen both in our households, but also in the infrastructure we use to e.g. travel from home to work or school and in the industrial facilities that produce what we need to have comfortable and healthy lives.
Even materials, which we believe to be resistant to corrosion like stainless steels, do corrode in certain circumstances. To protect materials from corrosion we first have to understand why it occurs after which we can find solutions to avoid it as much as possible.
Avoiding corrosion means we can save costs and in extreme situations even save lives.
worldstainless has two recorded webinars on the subject of corrosion available:
Chapter 1: https://youtu.be/0XYVQ5pelTU
Chapter 2: https://youtu.be/CPrwLxgxR0k
You can contact us at education@worldstainless.org with any questions you might have.
The corrosion resistance of stainless steel arises from a “passive”, chromium-rich, oxide film that forms naturally on the surface of the steel. The mechanisms of corrosion include crevice corrosion, pitting corrosion, intercystalline (or intergranular) corrosion (ICC), stress corrosion cracking (SCC) and galvanic (bi-metallic) corrosion and are often associated with chlorides or acid conditions.
Source: British Stainless Steel Association
The combination of tensile stress and a specific corrosive environment can crack stainless steels. This mode of attack is termed stress corrosion cracking (SCC). The most common environmental exposure condition responsible for SCC of stainless steels is the presence of chlorides. Although no stainless steel grade is totally immune to chloride SCC, the relative resistance of stainless steels varies substantially.
Source: Specialty Steel Industry of North America
Laboratory abrasive and abrasive-corrosive testing has been carried out on a range of ferritic, austenitic and martensitic stainless steels and the results compared with the testing of similar materials in situ in the abrasivecorrosive conditions of a gold mine. All grades were found to have better abrasive-corrosive resistance than proprietary abrasion-resistant alloys.
Download document hereComplex design requirements can make it necessary to combine different metallic materials within the same component. Also, chance combinations can often be found, governed only by the availability of, for instance, fasteners or shims. In certain circumstances, such mixed-material designs can lead to corrosion in one of the partner materials. This phenomenon includes galvanic corrosion, in which two different metals form a galvanic couple.
The present publication describes the principles of galvanic corrosion and the main parameters that allow designers to estimate corrosion risk.
This brochure is available in English, Czech, Dutch, Finnish, French, Italian, Polish, Spanish, Swedish and Turkish
[clicking on the language will open the pdf]
This handbook is designed to acquaint the reader with the 300 series stainless steels, particularly grades 304 and 316 and their applications in areas where coastal or salt corrosion is a factor in the life of a metal component.
Source: Specialty Steel Industry of North America
Corrosion by soil is a complex phenomenon due to the great number of variables involved. In principle, stainless steels should be in the passive state in soils, but the presence of water and aggressive chemical species such as chloride ions, sulphates and as well as types of bacteria and stray current, can cause localised corrosion.
Download this documentWhen two different metals are immersed in a corrosive solution, each will develop a corrosion potential. If the corrosion potential of the two metals is significantly different, and they are in direct contact and immersed in an electrolyte, the more noble metal will become the cathode and the more active metal will become the anode. A measurable current may flow between the anode and the cathode. The corrosion rate of the anode will be increased and the cathode decreased. The increased corrosion of the anode is called "galvanic corrosion".
Source: Specialty Steel Industry of North America
Stainless steels are susceptible to crevice or pitting attack in chloride bearing waters. Their behavior has been studied by a number of investigators. There is considerable variation in the percentage of apparently identical sites where attack occurs, when it occurs.
Source: The Hendrix Group, Materials and Corrosion Engineers
When used properly, stainless steel enjoys a strong and enduring reputation for visual appeal and structural integrity in a wide range of applications and environments.
But, like all materials, stainless steel may become stained or discoloured over time, impairing the overall look. This brown discolouration - tea staining - has been identified in coastal applications in Australia and overseas.
Source: Australian Stainless Steel Development Association
Pitting and stress corrosion can result from moist thermal insulation where chlorides are present. This information sheet provides background information on the sources of chlorides within such insulation materials and describes two corrosion prevention methods.
Source: British Stainless Steel Association
The paper describes an investigation which seeks to understand the corrosion mechanisms of Aluminium, Magnesium Mild Steel and Stainless Steel in different cement systems.
Source: University of Sheffield
Please note this resource is not free
Read more hereA general textbook on all types of corrosion. Contains a chapter on metals and alloys including stainless steels (to order as a hard copy).
Source: Corrosion Doctors

